Penna Dexter
Two decades after the Clinton FDA approved the abortion pill, a lawsuit has been filed asking for the removal of these drugs from the US market.
Alliance Defending Freedom filed suit in federal court on behalf of four medical organizations and four doctors who had treated patients with these drugs. The Department of Health and Human Services is also named as a defendant.
The lawsuit claims that the FDA lacked the authority to approve the two-drug regimen known as chemical abortion and did not adequately study its safety. Chemical abortion is four times as dangerous as surgical abortion. The FDA has been notified of at least 20 maternal deaths and over 2000 severe complications resulting from chemical abortion.
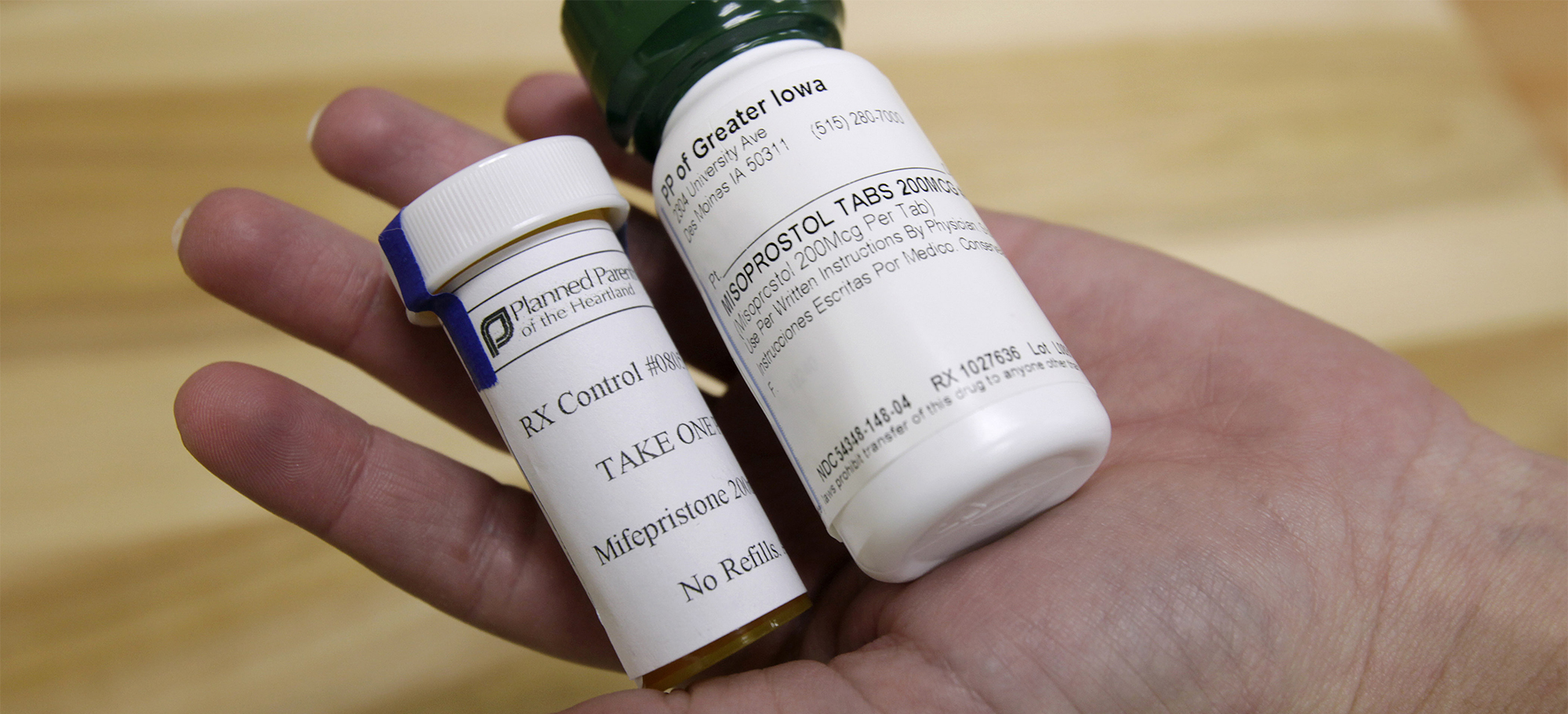
The first drug a woman takes in a chemical abortion is mifepristone — brand name: Mifeprex. It blocks the hormone progesterone, causing the baby’s death, and can have serious side effects, including hemorrhaging, immune system inhibition, and septic shock. The second pill, misoprostol, brings on contractions that cause the woman to expel the baby from her body, usually alone in her bathroom.
The lawsuit also challenges the FDA’s progressive easing of restrictions on chemical abortion. In 2000, the agency approved the 2-drug regimen to be used during the first seven weeks of pregnancy and then, in 2016, extended it to be used up to 10 weeks gestation. The expedited process used to approve the drugs is meant to speed therapies for life-threatening illnesses. As ADF Senior Counsel, Julie Marie Blake points out, “Pregnancy is not an illness.”
Over time, the FDA has also removed the requirement that a woman be examined in person when receiving a prescription for chemical abortion drugs.
Chemical abortions account for more than half the abortions done in the US. With the end of Roe, requests are skyrocketing. The lawsuit asks the court for a preliminary injunction to stop the sale of abortion pills while the case moves forward.
It’s a serious and needed effort. 
 Listen Online
Listen Online Watch Online
Watch Online Find a Station in Your Area
Find a Station in Your Area









 Listen Now
Listen Now Watch Online
Watch Online
